Organic Chemistry - Aromaticity and Benzene Rings
Audio Brief
Show transcript
This episode explores aromatic compounds, focusing on benzene's unique structure, exceptional stability, and characteristic reactivity.
There are three key insights from this discussion.
First, aromatic compounds like benzene exhibit exceptional stability from a cyclic, planar system of delocalized pi electrons, quantified as resonance energy.
Second, benzene's stability favors substitution reactions, preserving its aromatic system, rather than typical alkene addition reactions.
Third, substituents on the benzene ring significantly impact its electron density and reactivity at various positions.
Benzene has a planar, cyclic six-carbon ring with all carbon-carbon bonds identical and of bond order 1.5. This delocalization provides exceptional stability, quantified by a resonance energy around 151 kilojoules per mole.
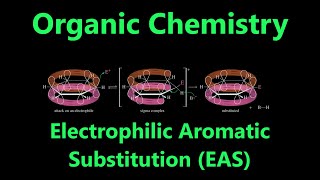
Its exceptional stability makes benzene unreactive toward typical alkene addition reactions. Instead, it preferentially undergoes electrophilic aromatic substitution. This preserves the stable aromatic system by replacing a hydrogen atom rather than breaking pi bonds.
Substituents on the benzene ring are electron-donating or electron-withdrawing. They influence the ring's electron density through resonance. This impacts its susceptibility to further reactions, directing new substituents to specific ortho, meta, or para positions.
Understanding these principles is key to the behavior and applications of aromatic compounds in organic chemistry.
Episode Overview
- Introduces aromatic compounds using benzene as the primary example, highlighting its unique structure and bonding.
- Explains the concept of resonance energy and demonstrates the exceptional stability of benzene through heats of hydrogenation.
- Contrasts the reactivity of benzene with typical alkenes, showing it undergoes substitution rather than addition reactions.
- Discusses how substituents on the benzene ring, categorized as electron-donating or electron-withdrawing groups, affect the ring's electron density through resonance.
Key Concepts
- Benzene Structure: A planar, cyclic, six-carbon ring with alternating double bonds where all carbon-carbon bonds are identical in length (1.397 Å) and have a bond order of 1.5. The delocalized electrons are often represented by a circle inside the hexagon.
- Aromaticity & Stability: Aromatic compounds like benzene are significantly more stable than predicted for a simple conjugated system. This stability is quantified by resonance energy, which for benzene is approximately 151 kJ/mol.
- Reactivity: Due to its stability, benzene is unreactive towards typical alkene addition reactions (e.g., bromination with Br₂, oxidation with KMnO₄). It preferentially undergoes electrophilic aromatic substitution, which preserves the stable aromatic system.
- Annulenes: A class of monocyclic hydrocarbons with alternating single and double bonds. Not all annulenes are aromatic; some are non-aromatic or anti-aromatic, indicating that a cyclic conjugated system alone is not sufficient for aromaticity.
- Substituent Effects: Groups attached to an aromatic ring can be classified as electron-donating (e.g., -OH, -NR₂) or electron-withdrawing (e.g., -NO₂, -C=O). These groups influence the electron density within the ring through resonance, affecting its reactivity.
Quotes
- At 00:21 - "it has a bond order of one and a half" - Explaining that the carbon-carbon bonds in benzene are intermediate between a single and a double bond due to resonance.
- At 02:52 - "it's down by 151 kilojoules. So that's quite a bit of resonance energy." - Quantifying the exceptional stability of benzene by comparing its predicted heat of hydrogenation to the actual experimental value.
Takeaways
- Aromatic compounds possess a special stability due to a cyclic, planar system of delocalized pi electrons.
- The bonds in a benzene ring are not alternating single and double bonds; they are all identical, hybrid bonds with a bond order of 1.5.
- Benzene's stability makes it resistant to addition reactions that would break the aromatic system; it favors substitution reactions instead.
- Substituents on a benzene ring can either donate or withdraw electron density, which changes the reactivity of the ring at specific positions (ortho, meta, and para).
- Resonance energy, calculated from heats of hydrogenation, provides a quantitative measure of the enhanced stability of aromatic compounds compared to their non-aromatic counterparts.