Work, Heat, and the First Law of Thermodynamics
Audio Brief
Show transcript
This episode discusses reversible and irreversible thermodynamic processes, specifically how to maximize work output.
There are four key takeaways from this discussion.
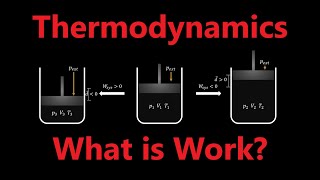
First, maximum work is extracted from a process only when it is performed reversibly. This means conducting the process infinitesimally slowly, maintaining continuous equilibrium, and ensuring internal and external pressures remain equal.
Second, the First Law of Thermodynamics, delta E equals q plus w, is a fundamental energy balance equation. It quantifies how a closed system's internal energy changes through heat and work transfer with its surroundings.
Third, adhering to correct sign conventions is crucial. Energy entering the system, such as absorbed heat, is positive, while energy leaving the system, like work done by the system, is negative.
Fourth, work and heat, both measured in Joules, represent different energy transfer modes. Work is directed energy, like pushing a piston, while heat is a disordered, random molecular transfer.
These principles provide a foundation for understanding energy transformations and maximizing system efficiency.
Episode Overview
- This episode explains the concepts of reversible and irreversible thermodynamic processes, focusing on how to maximize the work done by a system.
- It provides a step-by-step derivation of the formula for calculating the work done during an isothermal, reversible expansion of an ideal gas.
- The distinction between heat (random energy transfer) and work (directed energy transfer) is clarified, along with definitions for specific heat and heat capacity.
- The First Law of Thermodynamics is introduced for both isolated systems (energy is constant) and closed systems (change in internal energy equals heat plus work).
- The concepts are applied through practical examples, including calculating work for both ideal and real gases, and determining the change in a system's internal energy.
Key Concepts
- Reversible Process: A process conducted infinitesimally slowly (quasi-statically), ensuring the system remains in equilibrium at all times. This process maximizes the work output and is characterized by the internal pressure equaling the external pressure (P_int = P_ext).
- Irreversible Process: A process that is not carried out slowly, leading to dissipative losses and less than maximum work.
- Work (w): A form of directed energy transfer. For an isothermal reversible process of an ideal gas, the work done is calculated by the formula:
w = -nRT ln(Vf / Vi). Work done by the system on the surroundings is considered negative. - Heat (q): A form of random energy transfer. The amount of heat transferred can be calculated using the formula
q = mcΔT, wherecis the specific heat of the substance. Heat absorbed by the system is considered positive. - Internal Energy (E_sys): The total energy contained within a system, including kinetic and potential energies of its particles.
- First Law of Thermodynamics: A statement of the conservation of energy. For a closed system, the change in internal energy is the sum of the heat added to the system and the work done on the system:
ΔE_sys = q + w.
Quotes
- At 00:13 - "To make a process reversible, we must change the volume very slowly (what is known as quasi-static)." - This quote provides the fundamental condition for achieving a thermodynamically reversible process.
- At 06:31 - "...what differentiates them is that work is directed... while heat is random." - This statement highlights the core conceptual difference between work and heat as two distinct modes of energy transfer.
- At 08:48 - "For a closed system that is not isolated, the change in energy of the system is done through the transfer of heat (q) and work (w) with the surroundings." - This is the restatement of the First Law of Thermodynamics, which forms the basis for energy calculations in most practical thermodynamic systems.
Takeaways
- The maximum amount of work can be extracted from a process only when it is performed reversibly, meaning slowly and in continuous equilibrium.
- The First Law of Thermodynamics,
ΔE_sys = q + w, serves as a fundamental energy balance equation. It quantifies how a system's internal energy changes due to the exchange of heat and work with its surroundings. - It is crucial to use the correct sign conventions from the perspective of the system: energy entering the system (heat absorbed) is positive, while energy leaving the system (work done by the system) is negative.
- While both are forms of energy measured in Joules, work represents an ordered transfer of energy (e.g., pushing a piston), whereas heat represents a disordered, random transfer of energy at the molecular level.